Hydrogen is thought to be a highly efficient and an almost perfect solution for energy storage. And its importance is growing in light of the volatility of renewable energies. But the conventional and rather complicated hydrogen generation through solar energy and subsequent electrolysis reduces the efficiency of the process. An interesting alternative could be artificial photosynthesis, for which researchers all over the world are developing the methods. Success and efficiency both hinge on suitable materials for the cells. The technological battle for the best efficiency (which has doubled over the last years) is in full force.
Plants have long been able to convert sunlight into chemical energy through photosynthesis. Solar energy captured by technological means may indeed be available worldwide in abundance, but not all the time and everywhere. The development of solutions storing solar power in batteries or hydrogen has a long way to go, and this although any harnessed energy could be captured much better in the form of hydrogen than electrically. Professor Roel van de Krol, who heads the Solar Fuels Institute at the Helmholtz Zentrum Berlin (HZB), explained: “One kilogram of a fuel like hydrogen stores about 100 times as much energy as a comparable battery.” A particularly interesting storage solution is one based on nature itself: artificial photosynthesis, a technology that is being researched across the globe.
Artificial leaf produces hydrogen
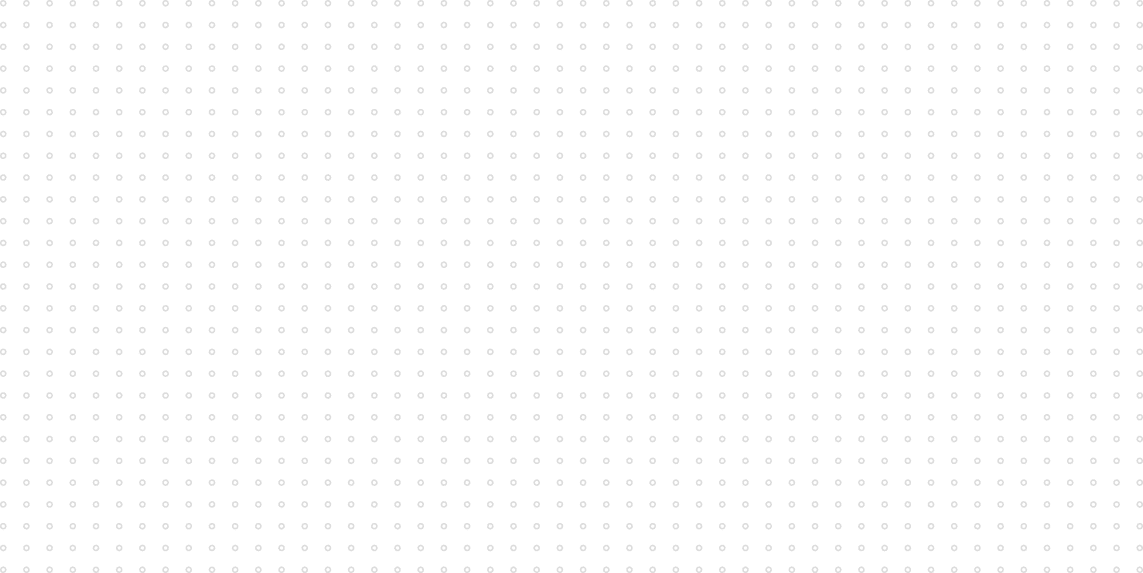
It has already been possible today to convert sunlight into chemical energy through artificial semiconductor systems. The “artificial leaf” required for the process consists of a solar cell, two electrodes and a membrane, combined with added functional layers. If immersed in water and put in sunlight, the harnessed electrical energy at the photoanode splits the water into oxygen atoms, protons and electrons, i.e., hydrogen. Whereas the oxygen remains in one chamber, the electrons and protons are led through the membrane into another, where a photoanode is used to combine them into hydrogen.
At present, there are still some issues to work out: Despite the additional layers of material, enough light must come through the solar cell to create the voltage for splitting water. Moreover, the semiconductor materials …
Above 15% efficiency
An international team made up of researchers from the Helmholtz Zentrum Berlin, the TU Ilmenau, the Fraunhofer Institute for Solar Energy Systems and the Joint Center for Artificial Photosynthesis (JCAP) of the California Institute of Technology has now succeeded in increasing the efficiency for direct solar water splitting a second time. The new benchmark value is 14 percent. The researchers used …
“Forecasts indicate that the generation of hydrogen from sunlight using high-efficiency semiconductors could be economically competitive to fossil energy sources at efficiency levels of 15% or more. This corresponds to a hydrogen price of about four US dollars per kilogram,” Professor Thomas Hannappel from the TU Ilmenau said. Professor Hans-Joachim Lewerenz from JCAP, who worked closely with the researchers from the HZB, added: “We’re nearly there. If we also manage to increase charge carrier mobility at the junctions a bit further, we could even use this semiconductor system to store more than 17 percent of the incident sunlight chemically as hydrogen.”
Search for inexpensive catalysts
In research, higher efficiency goes hand in hand with a search for inexpensive and efficient catalysts which are required at the anode for water splitting and the decrease of reaction times. So far, the process has used effective but expensive metals like platinum. Conversely, the artificial leaf contains a JCAP-developed catalyst that is cheap and consists of a two-nanometer-thick nickel layer to increase efficiency. Another 62.5 nm thin film of TiO2 prevents corrosion and improves the stability of the gallium-arsenic photoelectrode. A principal element of the JCAP development is the plastic membrane that keeps the oxygen and the hydrogen gases separate to prevent the creation of oxyhydrogen.
…
More layers = more capacity
There are yet other organizations which explore the potential of solar-based hydrogen production: Researchers from the Institute of Energy and Climate Research at the Jülich Research Center have developed a silicon-made multi-layer solar cell that can be produced quite cost-effectively and creates hydrogen based on artificial photosynthesis directly from sunlight. The efficiency achieved so far is 9.5 percent. “The difficulty, however, is to create a sufficiently high photovoltage. In practice, around 1.6 volts are necessary to kick-start the reaction for water splitting. Common crystal silicon solar cells, which have a photovoltage considerably lower than one volt cannot accomplish that,” explained Jan-Philipp Becker.
…
Algae and cyanobacteria
Researchers from the Center for Biotechnology at Bielefeld University are working on a way to utilize proteins from algae and semiconductor nanomaterials to capture the energy of the sun. This energy will then be used by the new artificially created catalysts to produce hydrogen. And researchers at the Hebrew University in Jerusalem have already coupled the light-absorbing parts of cyanobacteria with an electrode for biocatalytic power generation. By using a polymer and a nanoparticle made of platinum, they managed to link the light-harvesting complexes and reaction centers of cyanobacteria photosystems to an electrode through oxygen molecules. If sunlight enters a cell, the glucose molecules can convert it into gluconic acid with the help of special enzymes. The released electrons move to the electrode and can be fed into an electric circuit. At present, however, these bacteria only achieve a modest 1% efficiency.
Author: Edgar Lange, freelance journalist specializing in fuel cells and hydrogen





















0 Comments