The research alliance Campfire wants to decarbonize the maritime sector with the production of green ammonia and its application in emission-free drives.
With an innovative process, the carbon-free energy source is to be produced from air, water and locally generated renewable energies and used in the future as marine fuel, primarily over medium distances. Routes on the Baltic Sea are ideal for this. In this respect, it was obvious that the alliance of a total of 31 partners from research, industry and policy advice had come together in the coastal region.
The cheers were great when Campfire was selected last April as one of 20 innovation initiatives by the Federal Ministry of Education and Research for the “WIR! – Change through Innovation in the Region”. Finally, the practical implementation of the individual projects will receive up to EUR 15 million over the next five years. Good prerequisites for far-reaching innovations. Government investment could pay off in the future: These cutting-edge research projects involve no less than the development of new fields of technology and solutions for sustainable structural change in eastern Germany.
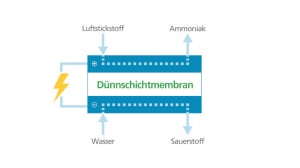
Campfire has presented a clear concept for the North-East model region, which extends from Rostock to Szczecin in Poland. The focus is on the decentralised storage of renewable energies and their utilisation in the transport sector. The production of green ammonia plays a key role here: NH3 is the perfect hydrogen storage medium and, due to its high hydrogen content of 108 g H2/l, has a volumetric energy density comparable to methanol. It is also easy to liquefy and can be safely stored, transported and handled according to experience in the chemical industry dating back more than a hundred years. Ammonia is the future – as a cost-effective fuel for emission-free shipping, as a raw material for sustainable fertilizers and for use in stationary energy supply systems.
So far, this recyclable material has only been produced industrially using the Haber-Bosch process. But the major disadvantage of this reaction process, which chemist Fritz Haber and entrepreneur Carl Bosch brought to market as early as 1910, is the enormous use of energy and fossil fuels. Since the bond between the two nitrogen atoms is extremely stable, it has to be broken under high pressure and high temperatures of up to 550 °C. In addition, most of the hydrogen required for synthesis is obtained from the methane contained in natural gas. Five percent of the natural gas produced worldwide and two percent of energy production is consumed to produce ammonia.
…
read more in H2-international July 2019



















0 Comments