Regarding fuel cells, a challenging and important issue are still the catalysts. To a considerable degree, they determine both the performance of stacks and their price. Currently, the most different nanoparticles are being examined in the most different structural combinations. This is also true for the field of water splitting, where catalysts are employed in electrolysis systems. The jury is still out on which materials could ultimately replace platinum in both cases, so work on the required catalyst quantity continues.
One of the potential contenders to replace platinum used in oxygen reduction is a nanoparticle mixture that is said to be less expensive but just as efficient as the original. The compound consists of a palladium-wolfram mixture at a ratio of one to eight, into which palladium islands of around one nanometer in size have been embedded. Thomas Wågberg from Sweden’s Umeå University explained that the mixture was similar in efficiency to a platinum catalyst because of its unique morphology, “but costs around 1/40,” as the palladium islands serve as high-efficiency catalytic hot spots. Together with his Swedish-Chinese team of researchers, Wågberg created the compound by employing a simple synthesis method that could also be used in a common microwave oven. He explained: “If we hadn’t used our argon as a protective inert gas, we could have created this great catalyst in our own kitchens.”
Meanwhile, American scientists have developed a new non-metallic catalyst system whose efficiency is almost as high during O2 reduction as the platinum one. The team of chemical engineers from the Wisconsin-Madison University said in the scientific research journal ACS Central Science that they had used a combination of nitroxyl and nitrogen-oxide mediators. Professor Shannon Stahl explained: “Despite the catalytic coupling having being used for aerobic oxidation previously, we did not know whether it would be a good fuel-cell catalyst. As it turns out, it is the most efficient molecular catalytic system ever to be reported.” [1]
The scientists at the Peter Grünberg Institute at the Research Center Jülich, on the other hand, have been trying to substitute part of the platinum with more inexpensive metals. Together with colleagues from TU Berlin, the researchers from the PGI had already had success in 2013 with creating particles that do not consist exclusively of platinum but of nickel or cobalt on the inside. These nanocrystals in the shape of octahedrons are said to require only a tenth of the usual platinum amount. They are created by first setting up a cross-shaped frame with six tips made of pure platinum. Then, the nickel or cobalt atoms bond with it until the gaps are filled and crystal growth stops on its own.
Ruhr University Bochum bets on carbon
In summer 2014, chemical engineers from the Ruhr University Bochum had already found a bifunctional catalytic material that can promote two opposite reactions: the cold combustion of hydrogen through oxygen in a fuel cell and the splitting of water by electrolysis. The material is carbon-based and incorporates manganese-oxide or cobalt-oxide nanoparticles. Professor Dr. Wolfgang Schuhmann and his team as well as Professor Dr. Martin Muhler “cut” the carbon nanotubes by using heat and oxygen in order to make use of the catalytic particles enclosed in them.
Gold nanoparticles
A*STAR in Singapore is yet another institute researching catalysts for electrolysis systems. While experimenting with water-resistant lanthanum-strontium-titanium-oxide, Andy Hor and his team discovered that nanoparticles made of gold can increase catalytic activity. The researchers added gold nanoparticles enriched with electrons to the compound. This resulted in better light concentration, which in turn accelerated catalytic reactions. The porous structure of the catalyst also increased overall surface area (one gram has a surface area of 100 m2). Hor explained: “The large surface area plays an important role in increasing photo-catalytic activity.”
Slow synthesis
In Germany, researchers are more focused on considerably cheaper materials, namely an iron-nickel-oxide compound. It was reported that the compound was used to synthesize nanoparticles that were ten times as efficient as everything described in the literature about attempts with comparable nickel-iron-based bonds. As stated by the Ludwig Maximilian University of Munich in May 2015, the team of Professor Thomas Bein and Professor Dina Fattakhova-Rohlfing relied on an intentionally slow synthesis. Ksenia Fominykh: “Common synthesis methods always create particles in the state with the greatest thermo-dynamical stability. Our method places the importance on an intentionally slow reaction time. It enables the establishment of less stable, so-called metastable states, with one of the results being the formation of distinct mixture ratios of nickel and iron in our nanoparticles.”
Together with colleagues from the MPI Düsseldorf and the Free University of Berlin, the scientists from Munich were successful in fully deciphering the extraordinarily small crystalline structure of the particle (1.5 nanometers). The discovered coupling of a very highly catalytically active surface area and a high crystallinity of the nanoparticles was one of the factors responsible for the structure staying intact even after sustained electrolysis under electric currents at high voltage. Additionally, it was said that the nanoparticles were easy to create and inexpensive to use for various applications.
Reduced platinum quantity
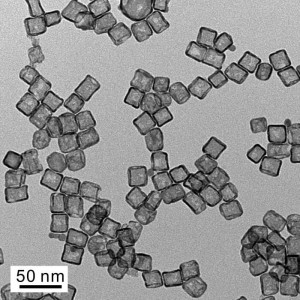
Another way to save costs is to reduce the platinum quantity required. Younan Xia from the American Georgia Institute of Technology has just recently made a breakthrough here: As reported in the magazine Science, Xia developed miniature platinum cubes whose nanostructure promises an up to seven times more efficient catalytic reaction at the same material cost. The cubes, which are only 20 nanometers in size (see figure 1), are hollow and possess ultra-thin walls of few atomic layers, providing a large surface area despite the small quantity of material.
The researcher created the nanocubes by letting individual atomic layers of platinum grow on the palladium nanocrystals and etch them away thereafter. The good controllability of the process enables the creation of one, two or three atomic layers. Until now, similar cubes had around 20 layers, which required much more platinum. Xia explained his motivation to create hollow platinum cubes as follows: “We did not want to waste material that takes up space inside but does not contribute to catalytic activity.”
[1] Gerken, J. B., Stahl, S. S., High-Potential Electrocatalytic O2 Reduction with Nitroxyl/NOx Mediators: Implications for Fuel Cells and Aerobic Oxidation Catalysis, ACS Central Science
DOI: 10.1021/acscentsci.5b00163 (2015)




















0 Comments